It allows the production of proteins with superior purity, including:
Technology
X’PROCHEM relies on an automated molecular assembly platform. Each protein is assembled amino acid by amino acid, thanks to a fully controlled production chain: a more precise and versatile technology which surpasses the limitations of conventional chemical and biological production methods.
It offers broad possibilities for optimizing proteins in order to maximize their therapeutic potential :
- Lengths up to 400 amino acids
- Native or modified proteins
- Complete control over protein structure
- High purity guaranteed :
- Without isoforms
- Without residues
- Without biological contaminants
- Without endotoxins
Robotic Molecular Assembly
Our innovative technology is based on a unique mastery of 100% automated molecular assembly processes, allowing the production of proteins, amino acid by amino acid.
1
Synthesis of the fragment: Amino acids are pieced together one by one to form the desired fragment.
2
Assembly of fragments: Synthesized fragments are assembled to obtain the desired protein.
3
Obtaining the linear protein.
4
Folding of the protein if necessary. The linear protein is folded to ensure its biological activity.
5
Obtaining the folded protein.
Exclusive and Patented Technologies
X’PROCHEM’s technology is built upon internationally recognized research efforts, safeguarded by a portfolio of patents and proprietary expertise.

CASE STUDY
Interferon Alpha-2a
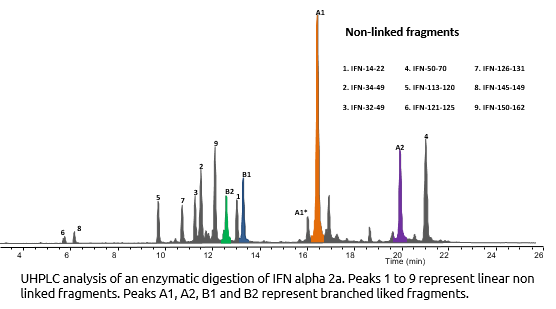
Interferon Alpha-2a (INF Alpha-2a) is a type 1 interferon that consists of 165 amino acid residues, which is a therapeutic mid-size protein, is traditionnaly produced by recombinant technology. This cytokine marketed under the brandname ROFERON-A®, is extensively used for its antiviral and antineoplastic properties.

Assembly strategy
We employed a 3-fragments assembly strategy in order to ensure optimized yield and purity. Fragments were synthetized by classical Solid Phase Peptide Synthesis with our proprietary resin and purified with RP-HPLC.
The assembly of the linear protein used our proprietary technology in a one-pot reaction, without any intermediary purification. Purification of the linear protein was done by RP-HPLC.
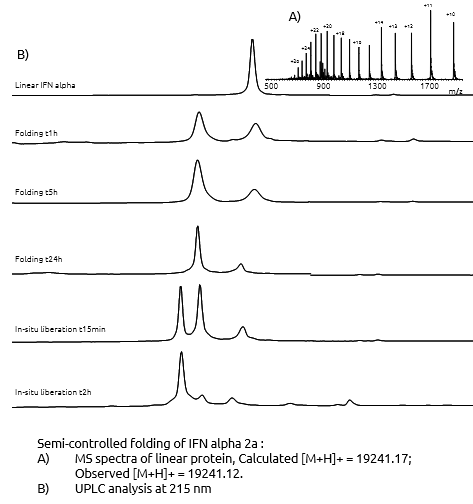
Folding of the protein
By using modification in the protein sequence, we performed a highly efficient folding in solution, leading to high yields. After completion of the folding step, the liberation of the native folded protein was done by in-situ treatment. The final purification of the protein was performed by RP-HPLC or HIC methods.
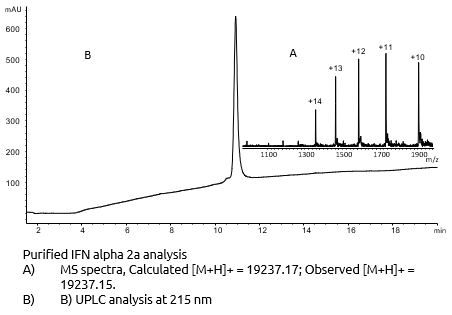
Final characterisation
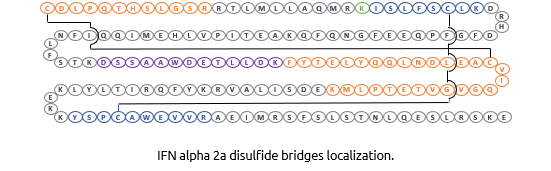
Structural characterisation
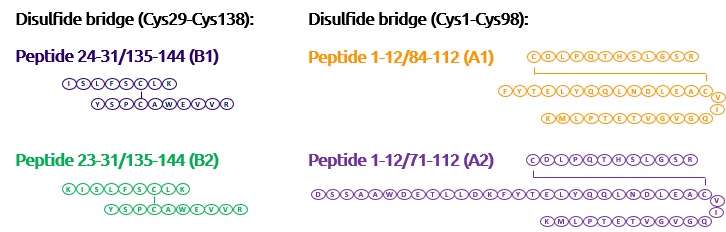
Interferon Alpha-2a is known to have 2 disulfides bridges: Cys1-Cys98 and Cys29-Cys138. We demonstrated the correct cysteines by enzymatic digestion (trypsine) and UHPLC/Mass Spectometry analysis of resulting fragments.